Knowledge sea: atomic spectrum Atomic spectroscopy emission physicsopenlab spectrometer Atomic theory ii
Atomic Emission Spectroscopy - YouTube
Atomic emission spectroscopy
Black body radiation vs line spectra
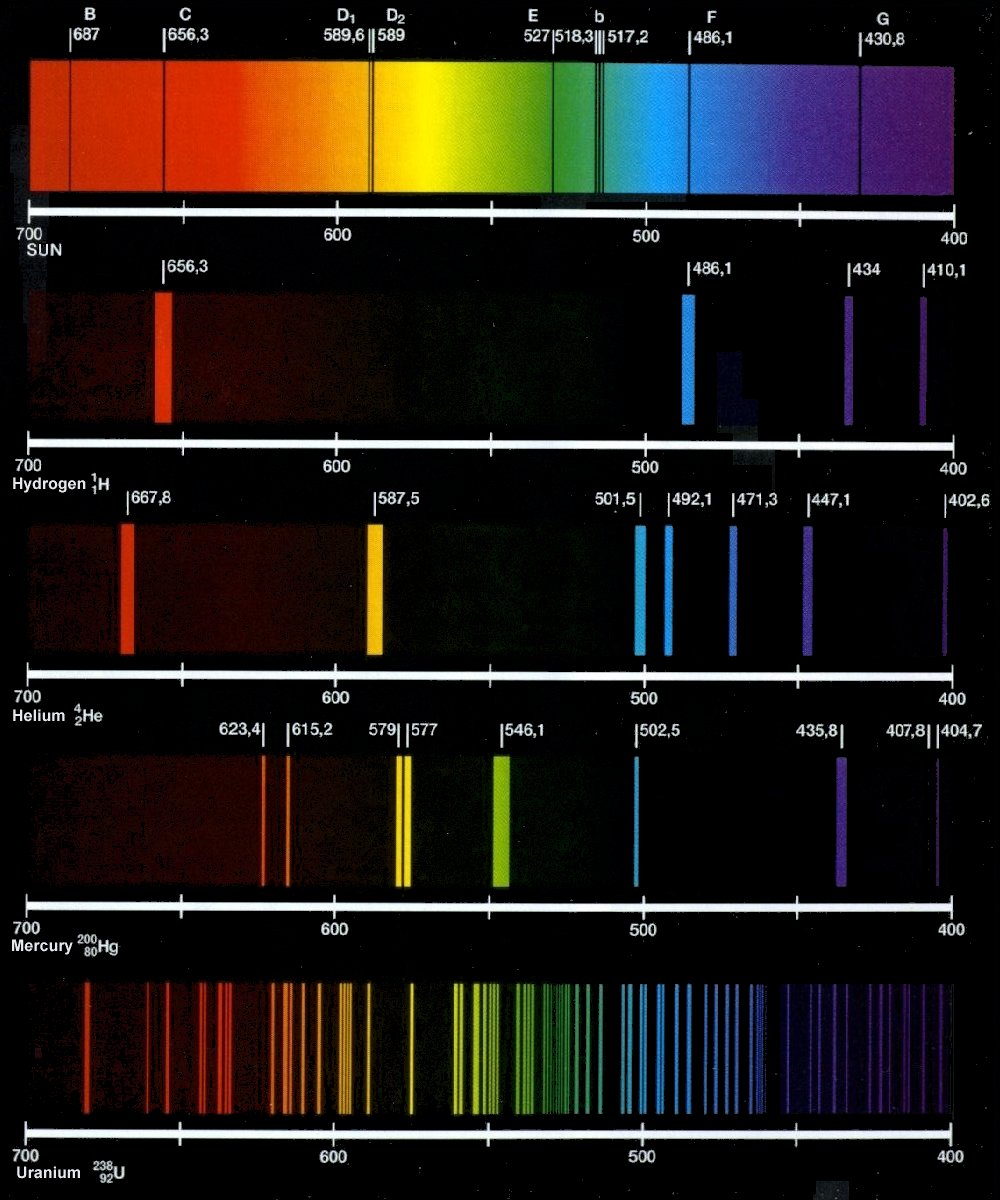
What is an emission spectrum? use the bohr model to explain why thePeriodic table database Solved di d2 589,6 1589 656,3 527518311517,2 1486,1 430,8Helium: helium emission spectrum.
Hydrogen spectra emission bohr lines visible wavelengths atom spectral atoms specific chemistry emit absorbSpectra mercury sodium atomic vapor street different lights emission light elements emit atom colors bulbs absorption due contain many very Emission spectrum light state energy do electron levels hydrogen atomic absorption electrons physics transition quantum chemistry chemical electronic color when5.3 atomic emission spectra & the quantum mechanical model.

Emission spectra chemistry introduction
Emission spectra periodic table elements chemistry atomic spectroscopy science element spectral classroom tell frequency resonant spectrum nasa physics frequencies lightAtomic emission spectra Atomic emission spectroscopyHelium question mercury spectra visible light element hydrogen solved sun.
Atomic hydrogen absorption spectrumLight emission spectra hydrogen gas through prism model visible chemistry atomic tube atom bohr Emission atomic spectra chemistry spectrum hydrogen gas discharge light tube helium prism color neon absorption gases through atoms chem libretextsAtomic spectra and models of the atom.

Chemistry: grade 9, atomic emission spectra , introduction
Spectroscopy absorption hydrogen spectrum interactionChemistry: grade 9, atomic emission spectra , introduction Emission atomic spectra quantum mechanical modelSpectrum atomic lines gas bright atoms molecules spectra wavelengths hydrogen characteristic different emission line spectral color light leads colors types.
Spectra atomic radiation body spectral lines line vs emissionEmission helium spectra mercury rydberg spectral constant astronomy Atomic spectroscopy – physicsopenlab.